Chemical Science & Engineering Research
Title
Kinetic, Equilibrium and Thermodynamics Study of the Adsorption of Pb(II), Ni(II) and Cu(II) Ions from Aqueous Solution using Psidium Guajava (Guava) Leaves
Authors
Nasiru Pindiga Yahaya,* Abdulsalam Umar, Yakong David Madugu and Adamu Abubakar
Department of Chemistry, Faculty of Science, Gombe state university, Nigeria.
*Corresponding author E-mail address: npy500@gsu.edu.ng (N.Y. Pindiga)
Article History
Publication details: Received: 06th April 2022; Revised: 11th May 2022; Accepted: 11th May 2022; Published: 24th May 2022
Cite this article
Pindiga N.Y.; Umar A.; Madugu Y.D.; Abubakar A. Kinetic, Equilibrium and Thermodynamics Study of the Adsorption of Pb(II), Ni(II) and Cu(II) Ions from Aqueous Solution using Psidium Guajava (Guava) Leaves. Chem. Sci. Eng. Res., 2022, 4(10), 9-16.
Abstract
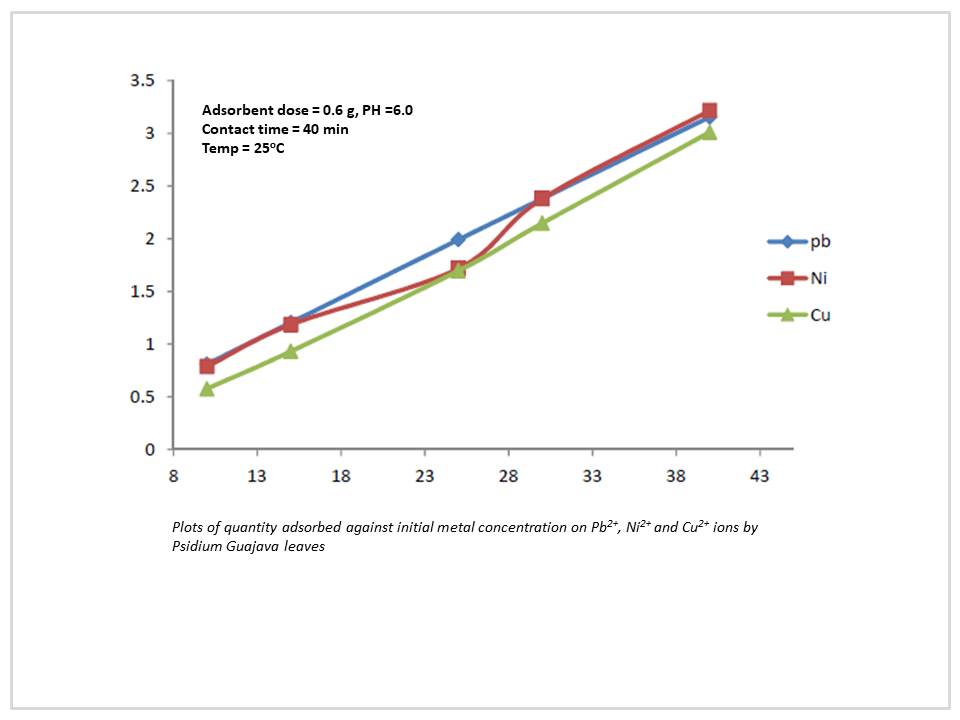
The kinetic, equilibrium and thermodynamic study of the adsorption of Pb2+, Ni2+ and Cu2+ ions from aqueous solution by the leaf biomass of Psidium Guajava (Guava) leaves were investigated at different experiment condition. Optimum condition of pH, Contact time, biomass dosage, initial metal ion concentration and temperature were determined, the maximum adsorption capacity was found to be 9.88, 9.5 and 4.21 mg/g, for Pb2+, Ni2+ and Cu2+, respectively. The kinetic studies indicated that the adsorption process of the Pb2+, Ni2+ and Cu2+ ions followed the pseudo-second-order model with R2 value of 1.0, 1.0 and 0.674 respectively. Equilibrium studies showed that the adsorption of Pb2+, Ni2+ and Cu2+ are well represented by both Freundlich and Langmuir isotherms with the Langmuir model given a better fit for Pb2+ with R2 value of 0.990 and Langmuir constant KL of 4.878, while Freundlich model was better fit for Ni2+ and Cu2+ ions with R2 value of 0.892 and 0.471 respectively, Freundlich constant Kf value of 1.8923 and 0.0196 and (ΔG0 -1705.02, -3745.18 and 401.18 KJmol-1) showed that the adsorption of pb2+ and Ni2+ are spontaneous, While Cu2+ are non-spontaneous. These finding indicate that the leaves biomass of Psidium guajava could be used for the adsorption of Pb2+, Ni2+ and Cu2+ ions from industrial effluents.
Keywords
Psidium Guajava (GUAVA) Leaves; Kinetic; Equilibrium; Thermodynamics Study; Adsorption of Metals