Applied Research Frontiers
Title
Synthesis and Evaluation of Coupler 4-Aryl-2-Aminothiophene-3-Carbonitrile and its Derivatives as Potential Coupling Component in Dye Synthesis
Authors
J. E. Ishegbe,* K. A. Bello, P. O. Nkeonye and A. A. Kogo
Department of Polymer and Textile Engineering, Ahmadu Bello University, local 810211, Zaira Nigeria.
*Corresponding author E-mail address: ishegbejoyce@gmail.com (J.E. Ishegbe)
Article History
Publication details: Received: 13th September 2021; Revised: 29th November 2021; Accepted: 20th December 2021; Published: 09th January 2022
Cite this article
Ishegbe J.E.; Bello K.A.; Nkeonye P.O.; Kogo A.A. Synthesis and Evaluation of Coupler 4-Aryl-2-Aminothiophene-3-Carbonitrile and its Derivatives as Potential Coupling Component in Dye Synthesis. Appl. Res. Front., 2022, 1(1), 1-6.
Abstract
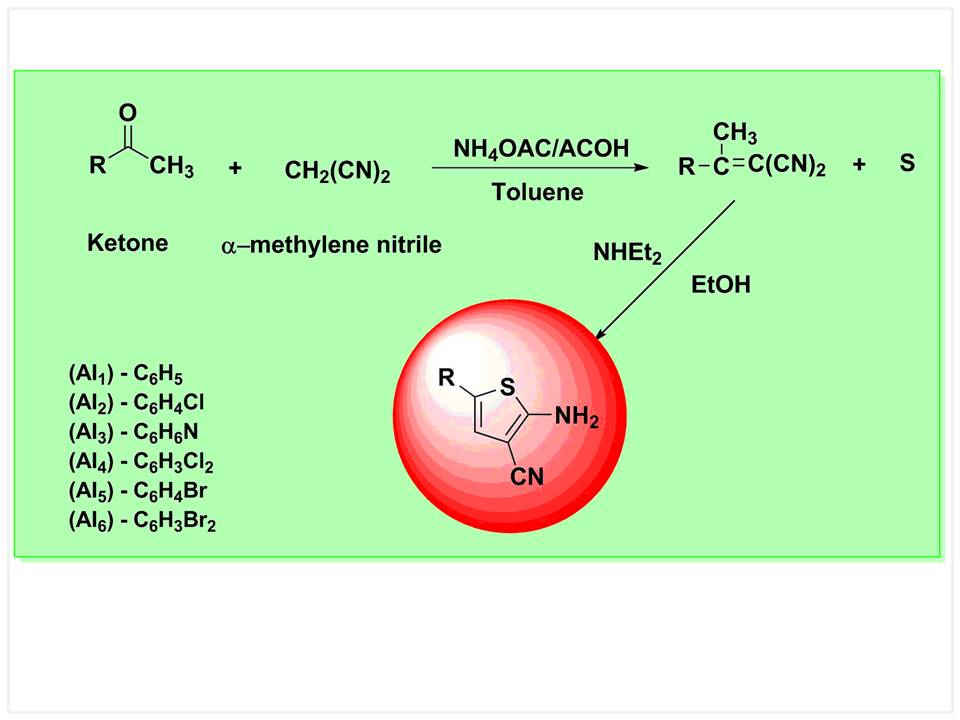
Thiophene nucleus has been established as the potential entity in the large growing chemical world of heterocyclic compounds possessing promising coupling characteristics. A series of coupler 4-aryl-2-aminothiophene-3-carbonitrile derivatives were synthesized. The synthetic method involves the reaction of ketones, aldehydes or 1,3-dicarbonyl species with activated nitriles and elemental sulphur in the presence of an amine base. Characterization of these coupling components was carried out by UV-Visible spectroscopy, Fourier-transform infrared spectroscopy (FT-IR), gas chromatography–mass spectrometry (GC-MS) and nuclear magnetic resonance spectroscopy (NMR) analysis. The synthesized compounds were purified, characterized and evaluated for spectroscopic properties. They were found to possess good coupling properties as well as high degree of brightness and a colour deepening effect compared to other heterocyclic couplers.
Keywords
Heterocyclic compounds; synthesis; absorption spectroscopy; NMR; Gewald reaction