Chemical Science & Engineering Research
Title
Kinetic, Batch Equilibrium and Thermodynamic Studies on Adsorption of Congo Red in Aqueous Solution onto Activated Carbon by H3PO4 Activation from the Hulls Seeds of Ziziphus Spina-Christi.
Authors
Bienvenu Hinimdou,a Massai Harounab and Raphael Djakba*a
aUniversity of Maroua, Faculty of Science, Department of Chemistry, Physical chemistry Laboratory, P.O. Box, 46 Maroua Cameroon.
bUniversity of Ngaoundere, School of Chemical Engineering and Mineral Industries, Laboratory of Physical chemistry, P.O. Box, 454 Ngaoundere, Cameroon.
*Corresponding author E-mail address: djakbakon@gmail.com (R. Djakba)
Article History
Publication Details: Received: 02nd Dec 2019, Accepted: 19th Dec 2019, Published: 28th Dec 2019
Cite this article
Bienvenu H.; Massai H.; Raphael D. Kinetic, Batch Equilibrium and Thermodynamic Studies on Adsorption of Congo Red in Aqueous Solution onto Activated Carbon by H3PO4 Activation from the Hulls Seeds of Ziziphus Spina-Christi. Chem. Sci. Eng. Res., 2019, 1(2), 1-8.
Abstract
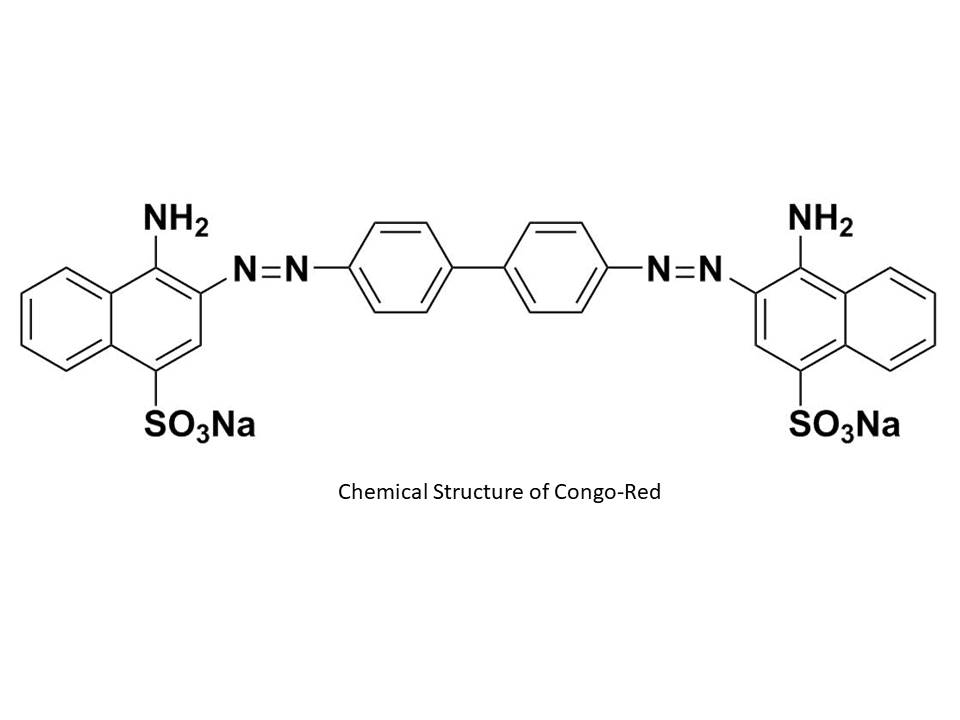
Pollution of the water environment of dyes can cause health risks to humans and environment. The management of these involves the establishment of specific methods. Herein, we demonstrated the adsorption capacity of Congo Red (CR) from an aqueous solution by activated carbon prepared from Ziziphus Spina-Christi shells. Prepared activated carbon was characterized by means of Fourier transform infrared (FTIR) spectroscopy and determination of the iodine number, and methylene blue index in aqueous solution. The experimental parameters such as pH of solution, contact time, adsorbent dose, initial concentration of CR and temperature were analyzed. To analyze the adsorption mechanism, Langmuir isotherms, freundlich models, Dubunin-Kaganer-Radushkevich (D-K-R), kinetic models of pseudo-first order, pseudo-second order, Elovich and Intraparticular Diffusion were studied. The adsorbent showed good potential for adsorption at pH 3.0 with an excellent yield of elimination of CR by active carbon (86.23% at 30°C). Adsorption equilibrium was reached after 20 min. Thermodynamic parameters such as ∆H° = -5.819 × 10-3 Kj/mol, ∆G° = -9.082 at -9.662 Kj/mol and ∆S° = 0.029 Kj/mol proved that adsorption mechanism of CR is possible, with the physisorption, spontaneous and exothermic in the ranges of temperature 30-60°C.
Keywords
Adsorption; kinetic; Congo-Red; activated carbon; Ziziphus spina-christi