Chemical Science & Engineering Research
Title
Competition Between Saturated and Unsaturated Components for Reacting with Emerging 1:1 Adduct Radical as Cause for Peaking Dependence of 1:1 Adduct Formation Rate on Unsaturated Component Concentration in Free-Radical Nonbranched-Chain Processes of Initiated Addition to Molecular C=C Bonds in Binary Systems
Authors
Michael M. Silaev
Chemistry Faculty, Lomonosov Moscow State University, Vorobievy Gory, Moscow 119991, Russia.
*Corresponding author E-mail address: mmsilaev@rc.chem.msu.ru
Article History
Publication Details: Received: 17th February 2020, Revised: 12th March 2020, Accepted: 12th March 2020, Published:18th March 2020
Cite this article
Silaev M.M. Competition Between Saturated and Unsaturated Components for Reacting with Emerging 1:1 Adduct Radical as Cause for Peaking Dependence of 1:1 Adduct Formation Rate on Unsaturated Component Concentration in Free-Radical Nonbranched-Chain Processes of Initiated Addition to Molecular C=C Bonds in Binary Systems. Chem. Sci. Eng. Res., 2020, 2(3), 7-11.
Abstract
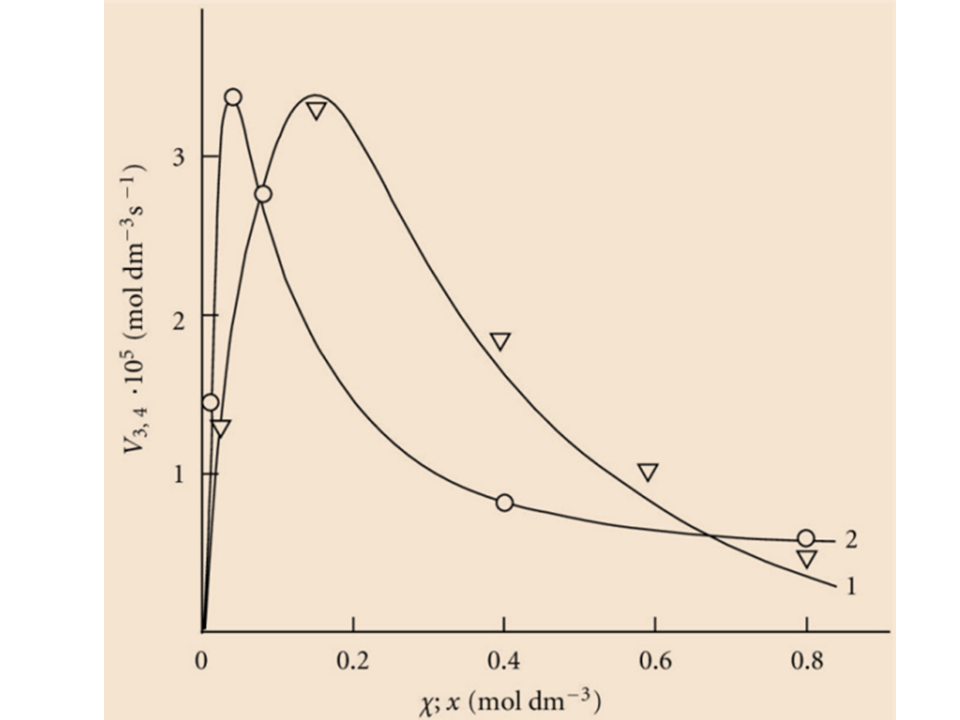
The reaction scheme is suggested for the initiated nonbranched-chain addition of free radicals to the double bonds of the unsaturated compounds. The proposed scheme includes the reaction competing with chain propagation reactions through a reactive free radical. The chain evolution stage in this scheme involves three types of free radicals. One of them is relatively low-reactive and inhibits the chain process by shortening of the kinetic chain length. Based on the suggested scheme, four rate equations (containing one to three parameters to be determined directly) are deduced using quasi-steady-state treatment. These equations provide good fits for the nonmonotonic (peaking) dependences of the formation rates of the molecular products (1:1 adducts) on the concentration of the unsaturated component in binary systems consisting of a saturated component (hydrocarbon, alcohol, etc.) and an unsaturated component (alkene, allyl alcohol, etc.). The unsaturated compound in these systems is both a reactant and an autoinhibitor generating low-reactive free radicals. It is shown that a maximum in the studied experimental curves arises from the competition between saturated and unsaturated components for reacting with the emerging 1:1 adduct radical.
Keywords
Binary System; Unsaturated Compound; Low-Reactive Radical; Autoinhibitor; Competing Reaction; Nonbranched-Chain Addition; Kinetic Equation; Rate; Parameters; Thermochemical Data; Energy