Title
5-(Phenyl-pyrrolidin-1-yl-methyl)-pyrimidine-2,4,6-trione: A Novel Corrosion Inhibitor for Mild Steel in Acidic Medium
Authors
K.P. Annamalai,*a P. Iniyavan,b V. Ashok Kumar,c M. Gopiraman*d and D. Tamilvendanc
aCAS-Haixi Institutes, Fuzhou, China - 350002
bJawaharlal Nehru Centre for Advanced Scientific Research, Bengaluru - 560064, India
cDepartment of Chemistry, National Institute of Technology, Tiruchirappalli - 620015, Tamilnadu, India
dDepartment of Applied Bioscience, College of Life & Environment Science, Konkuk University, Seoul - 05029, South Korea
*Corresponding author E-mail address: kpannamalai@outlook.com (K.P. Annamalai); gopiraman@konkuk.ac.kr (M.Gopiraman)
Article History
Publishing details: Received: 3rd November 2019, Revised: 3rd December 2019, Accepted: 27th December 2019, Published: 2nd January 2020
Cite this article
Annamalai K.P.; Iniyavan P.; Ashok Kumar V.; Gopiraman M.; Tamilvendan D. 5-(Phenyl-pyrrolidin-1-yl-methyl)-pyrimidine-2,4,6-trione: A Novel Corrosion Inhibitor for Mild Steel in Acidic Medium. Green Rep., 2020, 1(1), 1-7.
Abstract
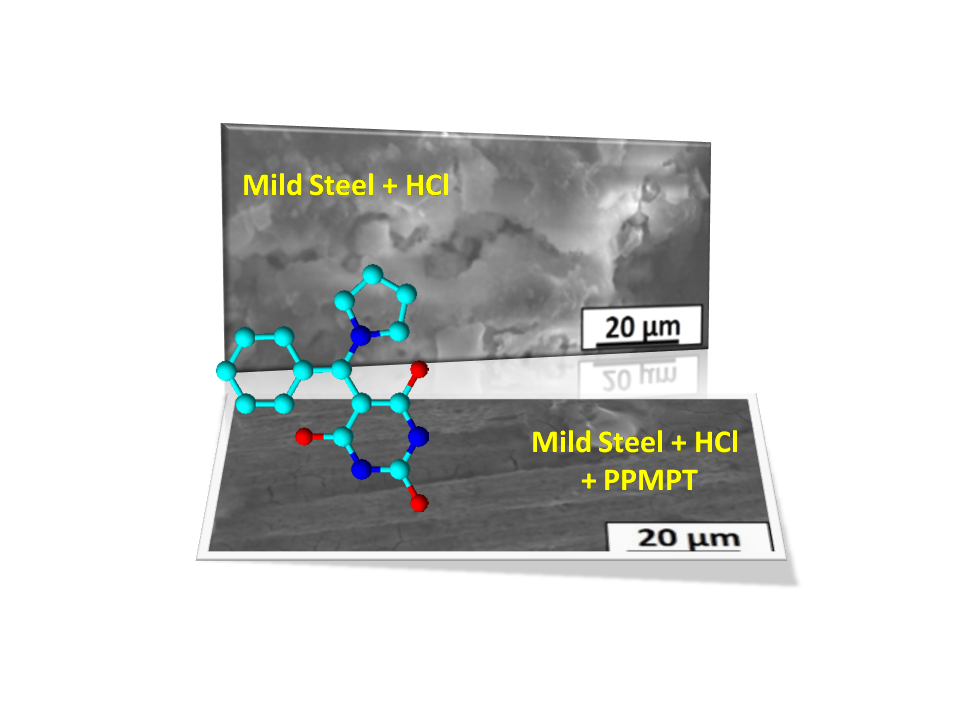
A new Mannich base, namely, 5-(phenyl-pyrrolidin-1-yl-methyl)-pyrimidine-2,4,6-trione (PPMPT), was successfully synthesized by simple green synthesis and the resultant PPMPT was used as corrosion inhibitor for mild steel in 1.0 M hydrochloric acid (HCl). The corrosion inhibition efficiency was studied by means of weight loss method and electrochemical techniques. The results demonstrated that 400 ppm of PPMPT has more than 95% inhibition efficiency against corrosion of mild steel in 1 M HCl. Calculated values of adsorption parameters such as ∆Hads and ∆Sads revealed a strong interaction between inhibitor molecules and mild steel surface in acidic medium. Negative value of ∆Gads indicates spontaneous adsorption of PPMPT molecules on the surface of mild steel. To further potentiodynamic polarization and electrochemical impedance measurements were also carried out to evaluate the type of inhibitor and corrosion inhibition mechanism, respectively. Ultraviolet–visible spectroscopy (UV-Visible), Fourier-transform infrared spectroscopy (FT-IR), scanning electron microscope and energy dispersion spectroscopy (SEM-EDS) were also carried out to support the inhibition efficiency of the PPMPT.
Keywords
Corrosion inhibition; Mild steel; Electrochemical impedance; Mannich base; Adsorption